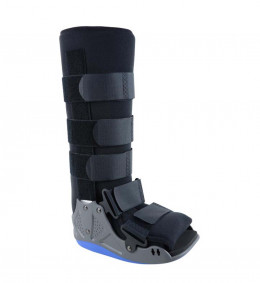
Adjustable lower leg walker LIGASTEP FIX WALKER 0800
Walking boot with rigid side uprights to immobilize and stabilize the lower third of the leg, ankle and foot.
Composition
Rigid components: polyamide, rubber, ethylenevinyl acetate, aluminum, thermoplastic polyurethane, polypropylene.
Fabric components: polyamide, polyurethane, polyester.
Properties
Immobilizes and stabilizes the lower third of the leg, ankle and foot. 90° rigid uprights on both sides Designed to ensure minimal dorsiflexion of the metatarsal bone. Curved, anti-slip sole that offers patients a natural, comfortable walking motion. Product can be worn on either foot.
Indications
Ankle/foot post-operative/post-traumatic immobilization.
Severe ankle/foot sprain.
Stable fracture of the lower third of the leg (tall version).
Conservative treatment of ankle/foot fracture.
Ankle/foot severe tendinopathy.
Conservative treatment of Achilles tendon rupture.
Contraindications
Do not use the product if the diagnosis has not been confirmed.
Do not apply the product in direct contact with broken skin.
Do not use in the event of known allergy to any of the components.
Do not use in the event of unstable fractures or fractures of the upper part of the tibia or fibula.
Do not use for patients weighing > 113 kg.
History of venous or lymphatic disorders.
Precautions
The systematic use of a long sock is recommended when wearing the device.
Ensure correct position and avoid folds when placing foam liner in the device.
Check the condition of the affected limb daily (with particular attention for patients with sensory deficit).
Do not bring the product into direct contact with greasy substances (ointment, cream, etc.).
Do not bring the product into direct contact with a dressing.
In the event of discomfort, significant hindrance, pain, variation in limb volume, abnormal sensations or change in colour of the extremities, remove the device and consult a healthcare professional.
Do not wear the product in a medical imaging device.
Do not wear the product when driving a vehicle.
It is recommended to adequately tighten the device to achieve immobilisation without restricting blood circulation.
In the event of pain, tingling or numbness, loosen the straps or deflate the air pads and ask a healthcare professional for advice.
For hygiene and performance reasons, do not re-use the product for another patient.
Prolonged immobilisation can sometimes cause muscle weakness. When resuming walking, be careful of risks of falling.
Undesirable side-effect
This device can cause skin reactions (redness, itching, burns, blisters…).
Possible risk of back pain due to gait pattern modification.
| Manufacturer | Thuasne | ||||||||||
| Country of manufacture | France |